Common bean seed multiplication
In order to examine the interaction between bean genotypes and rhizobium strains nodulating bean, seed of 29 different bean varieties that represent a great genetic diversity were obtained from CIAT, Colombia. They were multiplied in a screenhouse on soil samples collected from potential bean growing areas in Sidama, Ethiopia.
5 kg of soil samples and 0.5 kg compost were thoroughly mixed, added into pots and well moistened for a week before transplanting germinated seeds. Two seedlings were planted per pot with 5 replications. All replications were kept together and the inner side of the screenhouse was sealed with insect net to avoid insect damage and cross-pollination.
The 29 genotypes had different rates of germination, ranging from 60% to 100%: 19 of them were climbing beans and 10 were bush beans. The maturity status (days from transplanting to harvesting) of the genotypes also varied and some genotypes, such as G20134, G1230, G24482, G19237A, G50545 and G1375, matured early (from April 10th-June 6th) (Picture 1c and d). Others, such as G830, G20141, G764, G11228, G772, G288A, G757, G50123, G8045, G810 and G19833, matured later (Picture 1a and b). Most of the late maturing genotypes among the climbing beans and early maturing ones were both from climbing and bush beans. The genotypes bore between 4 to 21 pods per plant and 1 to 7 seeds per pod. Climbing beans produced more pods per plant.

Isolation of common bean nodulating rhizobia
Rhizobium strains nodulating common bean were isolated from common bean growing areas of southern Ethiopia to complement the genetic diversity of the strains needed for genotype x genotype interaction study. Soil samples were collected from bean growing farms with history of no inoculation and rhizobia from the soil were trapped by Nasir, Ebado and Hawassa Dume bean varieties (Picture 2a). At the flowering stage the plants were carefully uprooted, nodules were collected and stored in plastic vials on silica gel (Picture 2b and c). Nodulation varied between the varieties and soil sites (data not shown).

Nodules stored on silica gel were imbibed in water for an hour and surface sterilized using 96% ethanol and 2.5-3% sodium hypochlorite according to the methods of Somasegaran and Hoben (1994) and Hungria et al. (2016). Surface sterilized nodules were crushed in sterile test tube and the suspension was streaked on Yeast Mannitol Agar containing Congo red (YMA-CR) (Picture 2d).
|
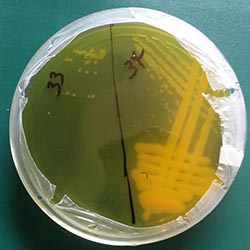
The isolated bacteria had pale pink colour on YMA-CR with entire margin and slightly raised elevation (Picture 2ef). Some of them produced sticky gums, which attach to a loop when touched. On YMA containing bromothymol blue (YMA-BTB), all the strains changed the media into yellow (Picture 3). This was also indicated by their growth on YMA-CR, as they grew within three days after plating out. These cultural characteristics of the isolates are typical features of fast-growing rhizobia. Ashenafi Hailu Gunnabo, Wageningen University, The Netherlands (Click here for his 2015 update) |
Picture 3: Strains growing on YMA-BTP |
References:
Somasegaran, P. and Hoben, H.J. (1994). Handbook for Rhizobia: Methods in Legume Rhizobium Technology. Springer-Verlag, New York.
Hungria, M., O’Hara, G.W., Zilli, J.E., Araujo, R.S., Deaker, R. and Howieson, J.G. (2016). Isolation and growth of rhizobia. In: Working with rhizobia, Howieson, J.G. and Dilworth, M.J. (Eds). Australian Center for International Agricultural Research (ACIAR). Murdoch University, Australia, pp.39-108.